Grantee Research Project Results
2010 Progress Report: Cardiovascular Toxicity of Concentrated Ambient Fine, Ultrafine and Coarse Particles in Controlled Human Exposures
EPA Grant Number: R832416C002Subproject: this is subproject number 002 , established and managed by the Center Director under grant R832416
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: Harvard Particle Center
Center Director: Koutrakis, Petros
Title: Cardiovascular Toxicity of Concentrated Ambient Fine, Ultrafine and Coarse Particles in Controlled Human Exposures
Investigators: Silverman, Frances , Gold, Diane R.
Current Investigators: Silverman, Frances , Gold, Diane R. , Urch, Bruce
Institution: University of Toronto
EPA Project Officer: Chung, Serena
Project Period: October 1, 2005 through September 30, 2010 (Extended to September 30, 2011)
Project Period Covered by this Report: August 1, 2009 through July 31,2010
RFA: Particulate Matter Research Centers (2004) RFA Text | Recipients Lists
Research Category: Human Health , Air
Objective:
The main objective of this project is to carry out controlled human exposures to both fine and coarse ambient particulate matter (PM) size fractions and examine acute changes in cardiovascular and respiratory outcomes. We hope to better understand how specific particle characteristics (size, composition, sources) affect health responses. The exposures are to concentrated ambient particles (CAPs) using our controlled particle exposure facility in Toronto, Ontario, Canada. Each subject has five 130-min exposures, assigned in random order, with a minimum 2-week wash-out period between. Exposures include 1 fine CAPs (250 µg/m3), 2 coarse CAPs (200 µg/m3), 1 HEPA filtered ambient air (HFAA) and 1 HEPA filtered medical air (HFMA). Cardiovascular and respiratory health outcomes are measured before, during and after exposures. Cardiovascular outcomes include: i) vascular dysfunction (brachial artery diameter and reactivity) by ultrasonography; ii) cardiac output by echocardiography; iii) blood pressure (BP) by automate arm cuff; iv) arterial pressure waveforms measures of large arterial compliance, central aortic BP and hemodynamics by SphygmoCor device; v) markers of systemic inflammation (CBCs and blood IL-6, CRP & endothelins); and vi) markers of oxidative stress in blood and urine. Respiratory outcomes include: i) pulmonary function by spirometry; ii) respiratory inflammation by induced sputum; iii) respiratory and nasal symptomotology; and iv) nasal inflammation by nasal smears, in a subset of allergic subjects. In addition, we will test for susceptibility genes (DNA methylation) of PM-induced oxidative stress in blood. During exposure, end-tidal CO2 is measured every 60 min using nasal prongs and beat-to-beat arterial BP is measured continuously for 10-min periods every 30 min using a Finometer finger cuff (pulse pressure) that includes calculated determinations of cardiac output, stroke volume and systemic vascular resistance. Mortara Holter (ECG) monitors are worn by the subjects over 24 hours, as a measure of cardiac autonomic dysfunction (HRV analyses). Exposures are characterized using continuous measures of particle mass (TEOM) and black carbon (PSAP) as well as integrated measurements (filter samples) of particle mass, sulphate, nitrate, ammonium, trace elements, organic and elemental carbon and biological material including airborne endotoxin and markers of fungi (β-(1,3)-D-glucan). On-site daily measures include vehicle counts, meteorological data, TEOM PM2.5, gaseous air pollutants (NO, NO2, SO2, CO, O3), as well as pollen characterization (GRIPST-2000 pollen sampler) and fungal spores/pollen (Burkard sampler). Daily stationary central site monitoring data (gaseous and PM criteria pollutants) are obtained to statistically adjust for potential affects on baseline pre-exposure data.
The specific hypotheses addressed by this project are the following:
- Acute human exposures to CAPs of coarse and fine size fractions result in cardiovascular responses including increased BP, vascular narrowing of the brachial artery diameter (BAD), vascular/autonomic dysfunction (impaired flow-mediated dilatation [FMD]), inflammation (respiratory and systemic), and oxidative stress compared to filtered air (control) exposures.
- Respiratory inflammatory responses (induced sputum), pulmonary function (flow-volume curves) and nasal/respiratory symptom responses will be greater with coarse CAPs than fine CAPs, compared to filtered air.
- Associations between CAPs and cardiovascular responses will differ by particle size fraction and PM composition.
Progress Summary:
Controlled Human Exposures: The first human exposures began late in November 2007. As of July 31, 2010, a total of 126 exposures have been completed. This includes 51 coarse CAPs and 26 fine CAPs, 28 filtered ambient air and 21 filtered medical air. A total of 36 subjects have been enrolled (met inclusion criteria) of which 3 did not qualify for the exposure phase of the study. Eight subjects who were enrolled dropped out after completing 0-2 exposures. Each exposure involves a 2-day protocol and we attempt to have two exposures each week, depending on subject availability and rescheduling due to upper respiratory tract infections (must be symptom free for 3 weeks), cancellations and holidays. Sixteen subjects have completed all five exposures. In addition, five subjects have completed four exposures and will do the fifth exposure. Subjects continue to be recruited and exposures carried out. The final goal is to have 25 subjects who have completed four to five exposures (four subjects did not complete the fifth exposure). At the earliest, the 25 subjects will be completed by late September/early October 2010.
Data Analyses:
Final analyses will be carried once the 25 subjects have completed their exposure testing, results are compiled, data have been entered and quality control checks carried out. Manuscripts then will be prepared.
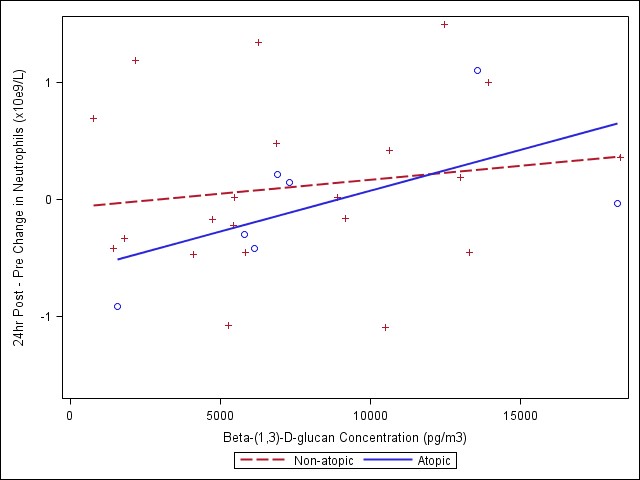
Meetings began in early 2009 to initiate collaboration between Harvard and the Gage with respect to Behrooz Behbod, a PhD student under the supervision of Diane Gold, interested in the inflammatory exposure data as part of his doctoral thesis. In June 2009, weekly teleconferences were established and interim data sets/variable labels sent to Harvard. His PhD proposal was finalized recently and will include analyses of respiratory and systemic inflammatory markers. Specifically, he will examine induced sputum leukocytes, CBCs and blood IL-6 and CRP. He also will examine health effect associations with endotoxin and β-(1,3)-D-glucan comparing the coarse and fine PM size fractions.
Data sets are updated in batches, once sufficient data are collected, and will be used to prepare abstracts/posters for scientific meetings. Interim statistical analyses are carried out on each data set, but will be merged into one large dataset once the study is completed. Statistical analyses are carried out using the SAS Mixed procedure with a random Subject Effect and exposure type as a four-level categorical Fixed Effect (coarse CAP, fine CAP, filtered ambient air and filtered medical air). Exposure means are presented as least square means estimates ± standard errors (SE). Comparisons between exposures (e.g., filtered medical air vs. fine CAP) are presented as differences of least squares means estimates ± SE. An initial analysis was carried out to test for significant differences between the first coarse CAP exposure (coarse-1) and the second course CAP (coarse-2) that each subject received. For a given outcome variable, if coarse-1 and coarse-2 were significantly different, they were treated as separate exposures in the analyses. Data that were not normally distributed were analyzed using the nonparametric Wilcoxon Signed Rank test. Due to batch compilation, the total number of subjects/exposures in each data set may differ. A summary of the findings follows.
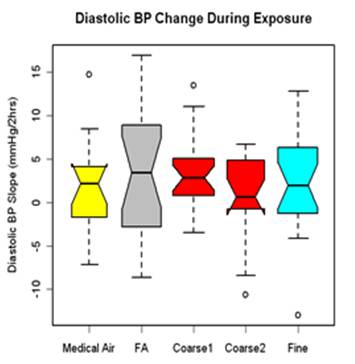
Blood pressure (BP) was measured in resting and seated subjects during exposure at the start (0-hr) and at 30-min intervals during the 130-min exposure (5 time points) using an automated BP arm cuff placed over the left arm brachial artery. Three measures were taken at each time point, 1 minute apart, and the average of the second and third measures averaged. Linear regression was used to fit a line over the five time points. Results are presented as the rate of change in BP over the exposure for 23 subjects and 99 exposures. Diastolic BP increased during exposures with similar responses for all four exposure types (figure 1). Only the coarse CAP response showed a significant increase (median change for all coarse CAPs: 2.49 mm Hg, p=0.08, n=20); however, this response was not significantly different from either the FA or medical air responses.
| Mass Concentration | Method |
| TEOM | real-time |
| Gravimetric | 47 mm Teflon |
| DustTrak | real-time |
| | |
| Composition | |
| Inorganic Ions | Environment Canada (EC) - IC, 47 mm Teflon filters |
| Carbon | EC - TOT, OC/EC, 25 mm Quartz filters |
| Black Carbon | PSAP - continuous |
| Metals | with Harvard samples at DRI - XRF, 37 mm Teflon filters |
| Selected Organics | e.g. PAH, motor vehicle tracers, EC - GC-MS, 25 mm Quartz filters |
| VOCs | Multi-bed thermal desorption tubes |
| Sources | |
| Motor vehicle tracers | see selected organics |
| Back trajectory analyses | Identify the path(s) of air masses and potential sources in the days prior to exposures |
| | |
| Biologic Components | |
| Endotoxin | Gage - J Scott, LAL assay, 25 mm polycarbonate filter |
| b-(1,3)- D-glucan | Gage - J Scott, Glucatell assay, 25 mm polycarbonate filter |
| Pollen/spores | Burkhard sampler, Gripst rotation impaction sampler - ambient levels |
| | |
| Ambient Gases | |
| NO, NO2, SO2, CO,O3, CO2 | Continuous gas analyzers |
Future Activities:
We will continue the exposure testing to complete the 25 subjects. Data reduction and QA/QC will be carried out and data sets updated and merged. Final statistical analyses then will be carried out. Some interesting findings will be further explored.
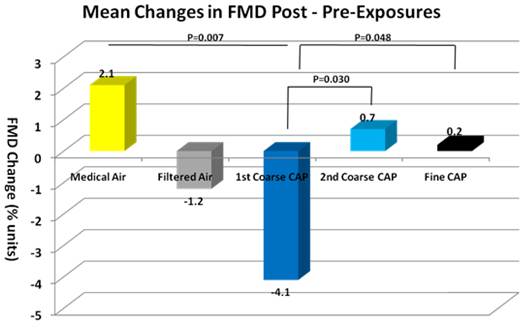
- First, we observed greater adverse responses to the first vs. second coarse CAPs exposures in FMD and some trends in other health outcomes, which were not explained by total mass concentration or elemental composition differences. If this were an adaptive response similar to that of O3, one would expect that the differences would be smaller the greater the time between the two coarse CAPs exposures. We will examine this and other possible explanations.
- Second, there was an apparent difference in responses between the filtered ambient air and filtered medical air, with the medical air showing the smallest changes. Thus, the medical air may be a more appropriate control, although the filtered ambient air response does suggest some effect of the ambient gases and volatiles that can pass through the HEPA filter. We have measured VOC exposure levels and gaseous pollutants and will explore potential associations.
- Third, we observed some interesting findings with the biologic data - endotoxin and β-(1,3)-D-glucan. This is an important component of the coarse CAPs exposures and few studies have considered that biologic components may contribute to respiratory and cardiovascular effects. We showed associations with both respiratory and systemic outcomes.
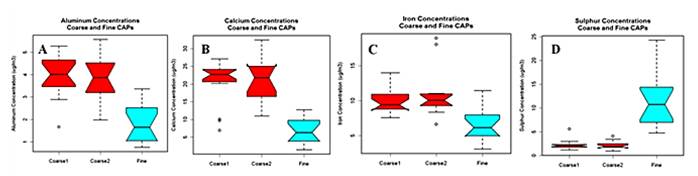
- Fourth, our exposure site has a significant contribution from traffic sources in close proximity to the CAP inlet; it thus provides the opportunity to explore specific associations using traffic markers of exposure. Specifically, we have obtained traffic counts, continuous black carbon measures and gaseous pollutants and will obtain motor vehicle tracers from filter samples as well as back trajectory analyses to determine sources during exposures. The fine CAPs exposures at higher concentrations than used in previous studies (250 μg/m3 vs. 150-200 μg/m3) does not appear to show more adverse responses, based on total mass concentration, but further analyses will be carried out to test for associations with individual constituents, as these may have varied across studies.
Once the exposure testing is completed, we will have data for more than 50 coarse CAPs exposures and with the continuation of the study (Health Canada) another 50 coarse CAPs. This will provide sufficient power to explore health effect associations in the largest coarse CAPs data set to date. Overall, the changes observed were small, but do provide important insights into the mechanisms of response to inhaled particles, including both respiratory and cardiovascular pathways. Furthermore, potential differences in response were shown between the coarse and fine PM size fractions. The final analyses will be the most comprehensive and it is hoped that they will uncover the most significant findings.
Journal Articles on this Report : 5 Displayed | Download in RIS Format
| Other subproject views: | All 8 publications | 5 publications in selected types | All 5 journal articles |
|---|---|---|---|
| Other center views: | All 206 publications | 199 publications in selected types | All 199 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Marsik FJ, Kamal AS, Kaciroti N, Harkema J, Corey P, Silverman F, Gold DR, Wellenius G, Mittleman MA, Rajagopalan S, Brook JR. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension 2009;54(3):659-667. |
R832416 (2009) R832416C002 (2009) R832416C002 (2010) CR830837 (Final) |
Exit Exit |
|
|
Fakhri AA, Ilic LM, Wellenius GA, Urch B, Silverman F, Gold DR, Mittleman MA. Autonomic effects of controlled fine particulate exposure in young healthy adults:effect modification by ozone. Environmental Health Perspectives 2009;117(8):1287-1292. |
R832416 (2009) R832416 (Final) R832416C002 (2009) R832416C002 (2010) R827353 (Final) |
|
|
|
Sivagangabalan G, Spears D, Masse S, Urch B, Brook RD, Silverman F, Gold DR, Lukic KZ, Speck M, Kusha M, Farid T, Poku K, Shi E, Floras J, Nanthakumar K. The effect of air pollution on spatial dispersion of myocardial repolarization in healthy human volunteers. Journal of the American College of Cardiology 2011;57(2):198-206. |
R832416 (Final) R832416C002 (2010) R832416C002 (Final) CR830837 (Final) R834798 (2012) R834798 (2014) R834798 (Final) R834798C002 (2014) R834798C002 (Final) R834798C004 (2012) R834798C004 (2014) R834798C004 (Final) |
Exit Exit Exit |
|
|
Thompson AM, Zanobetti A, Silverman F, Schwartz J, Coull B, Urch B, Speck M, Brook JR, Manno M, Gold DR. Baseline repeated measures from controlled human exposure studies: associations between ambient air pollution exposure and the systemic inflammatory biomarkers IL-6 and fibrinogen. Environmental Health Perspectives 2010;118(1):120-124. |
R832416 (2009) R832416 (Final) R832416C002 (2009) R832416C002 (2010) R832416C002 (Final) CR830837 (Final) |
|
|
|
Urch B, Speck M, Corey P, Wasserstein D, Manno M, Lukic KZ, Brook JR, Liu L, Coull B, Schwartz J, Gold DR, Silverman F. Concentrated ambient fine particles and not ozone induce a systemic interleukin-6 response in humans. Inhalation Toxicology 2010;22(3):210-218. |
R832416 (2009) R832416 (Final) R832416C002 (2009) R832416C002 (2010) R832416C002 (Final) |
Exit |
Supplemental Keywords:
concentrated air particles, acute cardiovascular effects, coarse particles, fine particles, vascular dysfunction, inflammation, oxidative stressProgress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R832416 Harvard Particle Center Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R832416C001 Cardiovascular Responses in the Normative Aging Study: Exploring the Pathways of Particle Toxicity
R832416C002 Cardiovascular Toxicity of Concentrated Ambient Fine, Ultrafine and Coarse Particles in Controlled Human Exposures
R832416C003 Assessing Toxicity of Local and Transported Particles Using Animal Models Exposed to CAPs
R832416C004 Cardiovascular Effects of Mobile Source Exposures: Effects of Particles and Gaseous Co-pollutants
R832416C005 Toxicological Evaluation of Realistic Emission Source Aerosol (TERESA): Investigation of Vehicular Emissions
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- Final Report
- 2009 Progress Report
- 2008 Progress Report
- 2007 Progress Report
- 2006 Progress Report
- Original Abstract
5 journal articles for this subproject
Main Center: R832416
206 publications for this center
199 journal articles for this center
