Grantee Research Project Results
Final Report: Anthropogenic influence on biogenic VOC oxidation: the role of NOx pollution in secondary organic aerosol production in the Southeast U.S.
EPA Grant Number: R835399Title: Anthropogenic influence on biogenic VOC oxidation: the role of NOx pollution in secondary organic aerosol production in the Southeast U.S.
Investigators: Fry, Juliane L
Institution: Reed College
EPA Project Officer: Chung, Serena
Project Period: April 1, 2013 through March 31, 2016
Project Amount: $299,995
RFA: Anthropogenic Influences on Organic Aerosol Formation and Regional Climate Implications (2012) RFA Text | Recipients Lists
Research Category: Air Quality and Air Toxics , Climate Change , Air , Early Career Awards
Objective:
Determine the reactive fate of NO2 in the summertime southeastern United States, with particular focus on the role of chemistry that results in secondary aerosol production, via organic (NO3 + biogenic VOCs) or inorganic (HNO3 uptake) mechanisms.
Summary/Accomplishments (Outputs/Outcomes):
Year 1 of the proposed work included field data collection and the beginnings of various analyses. The postdoctoral fellow, Dr. Ben Ayres, hired for Years 1 and 2 of this project, helped to quickly prepare for field deployment 3 months after the beginning of the grant period, and two Reed undergraduates (Danielle Draper and Hannah Allen) were hired to join Dr. Ayres and the PI (Fry) in the field for June and July 2013. As proposed, for the 6-week campaign we ran (1) a cavity ringdown spectroscopy (CRDS) instrument, borrowed from the lab of Steve Brown from National Oceanic and Atmospheric Administration (NOAA), to measure NO3 and N2O5; (2) a leased Monitor for AeRosols and GAses (MARGA) to measure inorganic gas and aerosol speciation; and (3) a NO3-initiated Potential Aerosol Mass (PAM) reactor in conjunction with the group of Jose Jimenez (University of Colorado-Boulder). These latter measurements represent a slight departure from the proposal; because the NO3/N2O5 CRDS instrument, when performing ambient monitoring, was not collocated with the Jimenez group Aerosol Mass Spectrometer (AMS) instrument, we had to move the CRDS instrument for the latter portion of the campaign to make the alternate measurements. Hence, we have 6 weeks of MARGA ambient measurements, 4 weeks of ambient NO3/N2O5 measurements and about 2 weeks of NO3 PAM measurements with NO3/N2O5 quantification by CRDS.
During Year 2 of the proposed work, Dr. Ayres and the PI continued work on analysis of the ambient dataset, which then was submitted for publication in Atmospheric Chemistry and Physics Discussions (APCD), and assisted the PI in mentoring undergraduates in the research lab during the 2014-2015 academic year. The PI also worked with both undergraduates previously hired by this grant (Allen and Draper) to develop papers submitted to ACPD in 2014/2015. Both now are Reed graduates, and have begun work on their Ph.D.s in atmospheric chemistry at the California Institute of Technology and University of California-Irvine, respectively. The research experience and introductions to other scientists in the field provided by this grant no doubt contributed to their decision to pursue graduate research in this area.
In Year 2, one new Reed undergraduate (Hyungu Kang, ‘15) was hired to join Dr. Ayres and the PI for a series of follow-up laboratory chamber experiments designed to complement and aid in interpretation of the field results, working with collaborators at University of Colorado-Boulder in July and August 2014. Kang also currently is working on a manuscript describing these follow-up experiments, and will begin graduate study in atmospheric chemistry in Fall 2015, at the University of Colorado-Boulder. Since the conclusion of his postdoctoral researcher contract, Dr. Ayres has taken an adjunct professor position at Portland State University teaching an undergraduate Air Quality course, and has worked with the Portland Department of Environmental Quality on measurements of airborne metals.
During Year 3, a second trip (Summer 2015) to University of Colorado-Boulder enabled additional follow-up experiments on NO3 + BVOC with collaborators in the Jimenez group, facilitating the laboratory interpretation of SOAS field results. For this study, an additional Reed undergraduate, Natalie Keehan (’15) was hired to accompany Fry and Kang to Boulder. Keehan currently is working as an analytical chemist at Emerald Performance Materials in Portland, OR. This additional visit also facilitated the PI to work with collaborators Jose Jimenez (CU Boulder) and Steve Brown National Oceanic & Atmospheric Administration Earth Sysytem Research Laboratory (NOAA ESRL) on currently ongoing analysis for which manuscripts are in preparation, analyzing NO3 + isoprene secondary organic aerosol (SOA) yields in the Southeast Nexus (SENEX) aircraft data. We accomplished the measurements required to complete all four goals of the original proposal, and have since completed all of the primary analysis of these datasets, as described below under headings for each grant goal. The PI continues to work on several ongoing additional analyses enabled by our participation in the SOAS campaign, also described below.
1. Determine NO2, N2O5, and NO3 measurement-based lifetimes of NO3 and N2O5 under the varying conditions of humidity and [BVOC] present at the field site. Determine the nightly variation – both within and above canopy – of the contribution of the anthropogenic oxidant NO3, derived from NO3, NO2, and O3 measurements. This will enable us to assess the anthropogenic (NO3) enhancement of the background (O3) oxidation of BVOC.
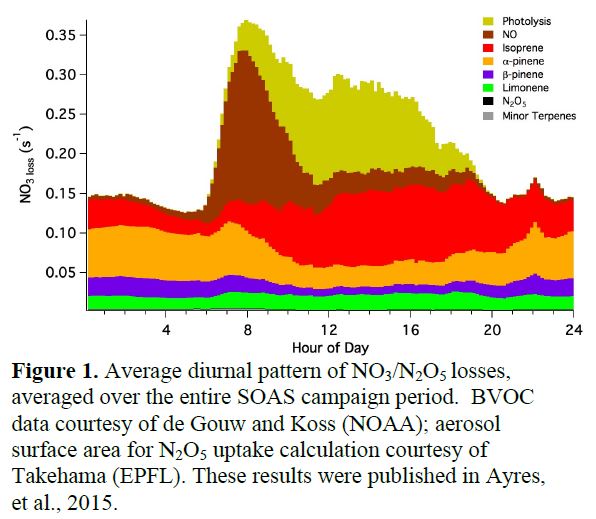
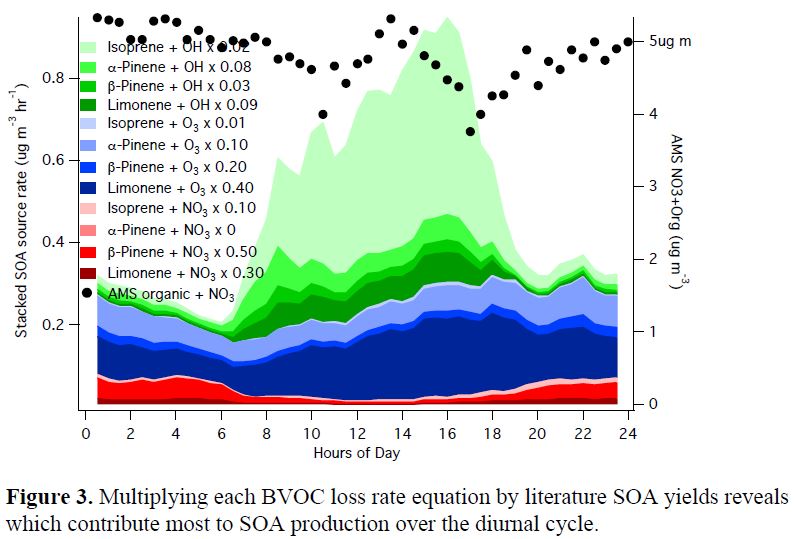
Figure 1 below shows the average diurnal cycle of NO3/N2O5 loss rates to the various BVOCs, compared to photolysis, NO reaction, and N2O5 heterogeneous uptake losses during the campaign. We find N2O5 aqueous uptake to be essentially negligible, despite high humidity and high particle surface area. As discussed below, we are finding evidence of other unexpected sources for aerosol-phase inorganic nitrates that will form the focal point of our analysis of the field data. However, using our NO3/N2O5 steady state analysis in conjunction with biogenic volatile organic compounds (BVOC) measurements from the NOAA group (de Gouw, Koss), we are able to demonstrate that NO3 + BVOC is the dominant sink of NO3/N2O5. This pathway is responsible for 50% of losses during the daytime when conventional wisdom would suggest that NO3 concentrations during the day approach zero and therefore would be unavailable to react with BVOC. These losses are a consequence of the high BVOC reactivity of the SOAS site. Similar O3-initiated rates can be calculated to directly assess the comparison to these NO3 lifetimes.
Turning this analysis to the other reactant, we investigated chemical losses to various oxidants from the standpoint of each measured BVOC. Figure 2 below shows the average diurnal cycle of speciated biogenic volatile organic compounds (VOCs) to NO3, OH and O3 oxidants, assessing the relative contribution of NO3 to BVOC losses, and finding this to be substantial, with ~1/3 of the monoterpene oxidation at night initiated by NO3. We multiply the concentrations and rate constant of each BVOC with the three major oxidants to see which oxidation reactions are responsible for BVOC loss rates.
This still crude analysis does not yet account for any change in boundary layer height or mixing at the top of the boundary layer. Still, it is provocative that integrating across the diurnal cycle would give a total production of 12 µg m-3 in a day, which, given the observed AMS loading of ~5 µg m-3, implies a daily loss of 7 µg m-3! Taking a literature particle deposition rate of −4.8 ng m−2 s−1 (the flux reported in Farmer, et al., 2013 for total nonrefractory PM1), with an average 1000 m boundary layer height, this is a deposition mass flux of -5 x 10-6 µg m-3 s-1. Integrating over 24 hours (= 86400 s), this would give a daily total deposition of about 0.4 µg m-3, substantially less than inferred here.
A clearly missing component is boundary layer dynamics, and so to move this analysis forward to developing a complete OA budget, PI Fry currently is working with Jordi Vila (Wageningen University) to apply a mixed layer model to interpreting the contribution of mixing, subsidence and advection. This work is ongoing and extends beyond the original goals of this grant, giving one example of how this grant will continue to pay dividends for Fry’s career.2. Determine near-surface levels of NO3 and N2O5, their sources and their sinks at several heights on the tower. These oxidant measurements will be conducted co-located with organic nitrate (R. Cohen / P. Wennberg) and possibly HNO3 (R. Cohen) measurements at various heights to assess local production of these higher NOy species from NO3 oxidation/N2O5 hydrolysis.
Figure 4 below shows measured N2O5 compared to a steady-state model, demonstrating that (1) we only rarely measured NO3/N2O5 above instrument detection limits due to rapid BVOC reactivity (see above), and (2) when N2O5 is detectable, its concentration matches the steady-state prediction well. The latter mitigates the problems of the former, because the observed correlations support the use of steady-state predicted NO3 production rate (from CRDS measured NO2 and O3) when [NO3] is below detection limits. Thus, we use the predicted production rate (Figure 5) for subsequent calculations. Note: because we observed very low/below detection limit ambient concentrations at the top of the measurement tower (where NO3 concentrations would be expected to be maximum due to distance from BVOC sources), we did not make any attempts to measure NO3/N2O5 at lower heights on the tower. All vertical gradient information will be gleaned via comparison to the aircraft vertical profiles.
As described in the Year 1 report and Ayres, et al., 2015, N2O5 hydrolysis was found to be a minor loss for NO3/N2O5 at this site. Using [NO3]ss from the above analysis in conjunction with measured monoterpene concentrations, cumulative nightly production of monoterpene organonitrates can be predicted and compared to available organonitrate measurements. This is shown in Figure 6a. We observe a strong correlation, suggesting that these NO3 + monoterpene reactions are responsible for producing a large fraction of observed aerosol organonitrate. This condensability of these organonitrates is further supported by the fact that most organic nitrate (red) is observed to be in the aerosol phase (blue), according to the Cohen group therma dissociation-laser induced flourescence (TD-LIF) measurements of total organic nitrates (Figure 6b).
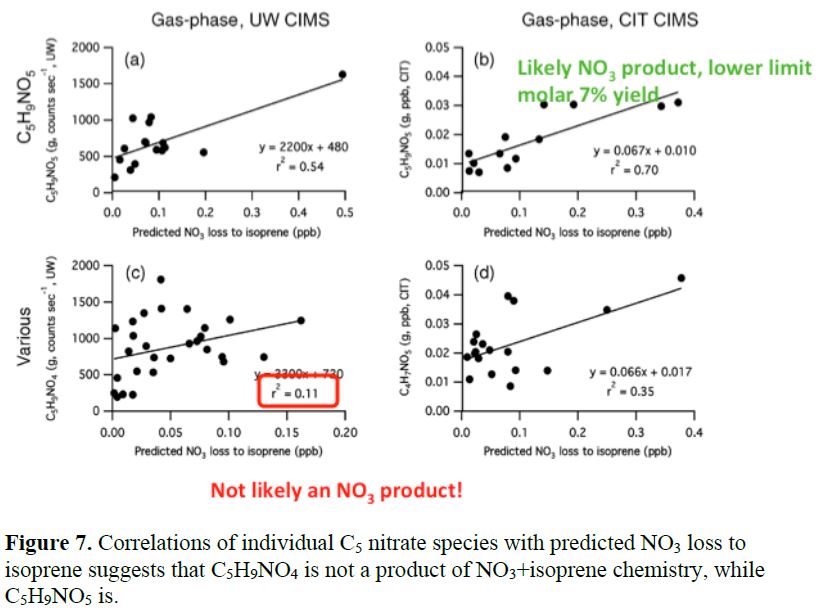
We also can correlate individual observed organonitrates with NO3 + monoterpene or NO3 + isoprene losses to assess which terpenes nitrate molecular formulae are sourced by each pathway. This analysis is described in detail in Ayres, et al., 2015, and sample figures are shown below. In Figure 7, we use correlation coefficients to determine that one molecule measured by the University of Washington Thornton group Center for Intelligent Materials and Systems (CIMS) is not likely a product of NO3 + isoprene chemistry, while another is.
2. (Continued) Nighttime P-3 vertical profiles as part of the SENEX campaign will provide context for the continuous measurements from the tower.
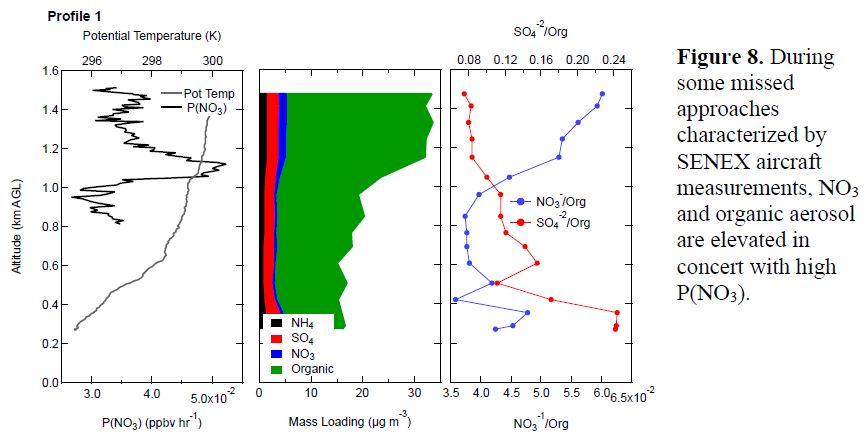
A similar NO3/N2O5 instrument was flown on the SENEX P-3 run by Pete Edwards and Steve Brown, which included three night flights during the SOAS campaign. This aircraft also had aerosol composition measurements by AMS. We have looked into how to best use these data in conjunction with the ground site data. A first analysis attempt in Year 2 was to investigate vertical profile data during missed approaches. This analysis sought to identify periods of SOA buildup by NO3 + BVOC chemistry in the nighttime residual layer. A preliminary look at a vertical profile during which NO3 to organic ratio and production rate of NO3 radical are both elevated aloft, taken during a flight over eastern Arkansas on 7/3/13 at 12:20 am, is shown in Figure 8.
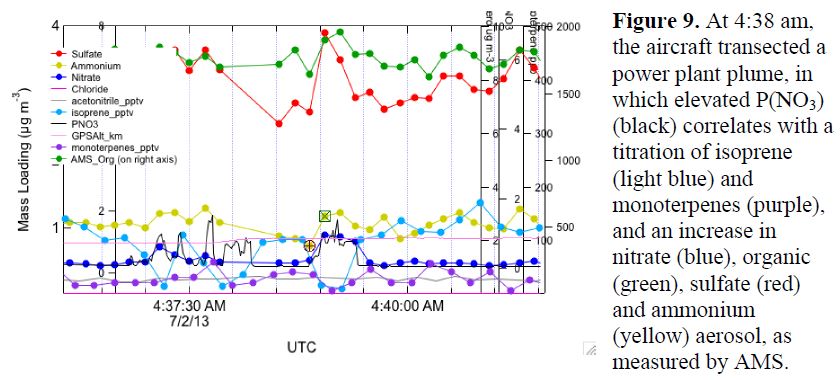
More recently, we have shifted gears to analyzing periods of level flight through power plant plumes, where production of NO3 radical (P(NO3)) is rapid, and the resulting titration of isoprene by NO3 enables an estimate of SOA yield from this process. This work is in process, in collaboration with Steve Brown and Pete Edwards at NOAA ESRL. An example time series transecting a power plant plume is shown in Figure 9.
3. Determine organic aerosol mass production in ambient air from NO3-oxidation in a Potential Aerosol Mass (PAM) flow reactor. By directly determining in-field SOA yields from ambient mixes of BVOCs, we will enable better understanding of organic aerosol formation from NO3-initiated chemistry, in comparison with other oxidants (J. Jimenez group will run OH and O3 PAM chambers as well).
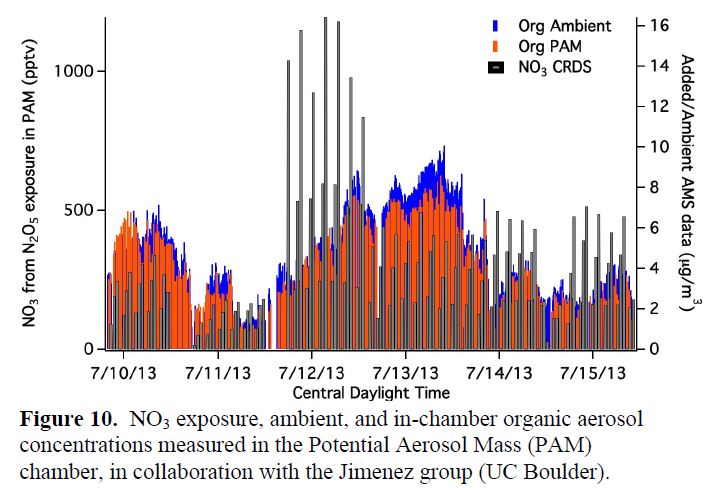
A NO3 Potential Aerosol Mass measurement was run for the last 2 weeks of the campaign, as described above. Moving the CRDS measurement of NO3 concentration down to the PAM chamber enabled online variation and measurement of NO3 concentrations into the PAM chamber. The chamber was run in a 2-hour cycle running through four NO3 exposure states: none, low, medium and high (Figure 10). In-PAM organic aerosol concentrations were alternately monitored with ambient concentrations to assess changes in aerosol loading due to oxidant exposure. Analysis of this organic aerosol production data is complicated by the fact that at SOAS, relative humidity was very high. We suspect that the walls of our PAM chamber became saturated with HNO3, such that any ambient NH3 encountered quickly yielded substantial inorganic nitrate aerosol. Because this wall artifact may be much larger than any actual ambient signal, we are not sure we will be able to learn much from the changes in aerosol observed in the PAM reactor, unfortunately. We did learn some valuable features of the PAM reactor itself. Our paper describing the BEACHON-RoMBAS use of the PAM chamber is forthcoming (Palm, et al., in prep).
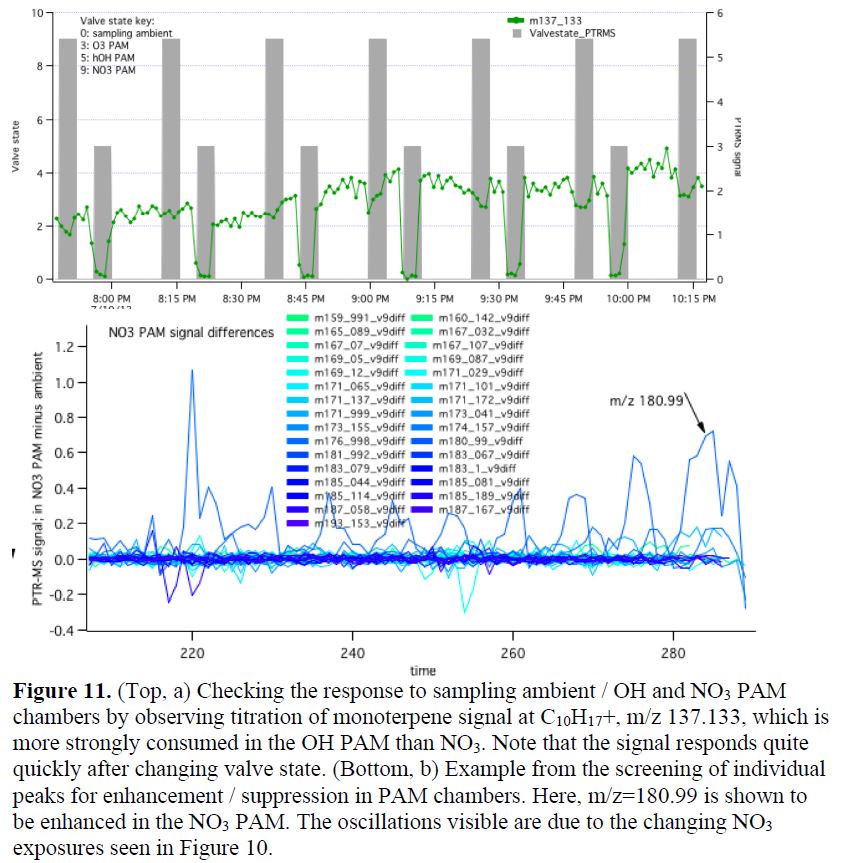
Most recently, I am revisiting this SOAS PAM dataset using Proton Transfer Reaction - Mass Spectrometry (PTR-MS) data collected by Lina Hacker and Astrid Kiendler-Scharr (Forschungszentrum Juelich), intermittently sampling ambient air and air treated with high concentrations of oxidants (in two PAM chambers, one each employing OH and NO3). This ongoing work is facilitated by my current sabbatical in Netherlands/Germany, which allows me to work closely with the group at FZ Juelich. The first step is to sort the PTR-MS data into periods corresponding to each oxidant (Figure 11a), which were cycled regularly during the campaign. Then, we screen all available assigned masses to identify those that increase under oxidation by a particular oxidant, identifying products of that particular reaction combination. An example is shown in Figure 11b, showing that m/z 180.99 is enhanced in the NO3 PAM.
Next steps will include assigning these peaks molecular formulae, and then using these identified peaks to inform a source apportionment analysis of ambient data collected throughout the SOAS campaign.
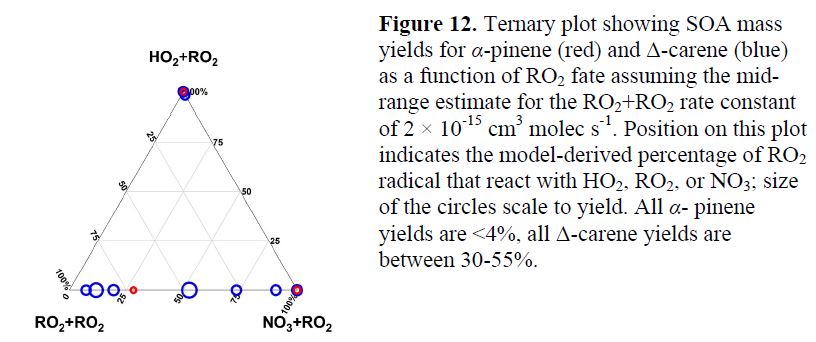
In the meantime, in order to complement the challenging SOAS NO3 PAM aerosol production analysis, we have undertaken a series of controlled chamber experiments investigating NO3 + monoterpene SOA formation mechanisms, as a collaborative effort with the Jimenez group at University of Colorado-Boulder. Similar to the PAM measurements, these may help interpret key aspects of field-observed NO3 SOA formation from SOAS. Shown below in Figure 12 is a summary of several recent laboratory chamber experiments conducted in collaboration with the Jimenez group, to complement and help to interpret the SOAS NO3 PAM observations. We sought to interpret the dramatic difference in nighttime NO3 radical initiated SOA yield observed from two dominantly emitted monoterpenes, α-pinene and Δ-carene, with very similar molecular structures. While α-pinene never yields much if any aerosol under chamber experiments across three regimes of potential nighttime RO2 radical fates (RO2, HO2, and NO3 as the next bimolecular partner for the initially produced nitrato-RO2 radical), Δ-carene consistently has high SOA mass yields. This suggestion of RO2-fate-independent SOA yields is encouraging to the application of chamber-based yield measurements to interpreting field data. This work currently is the subject of a manuscript in preparation (Kang, et al., 2016).
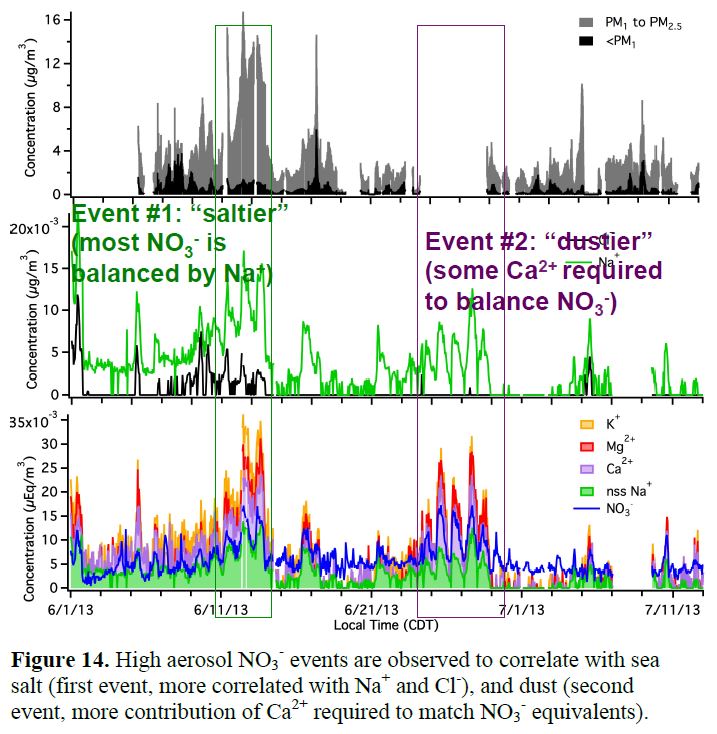
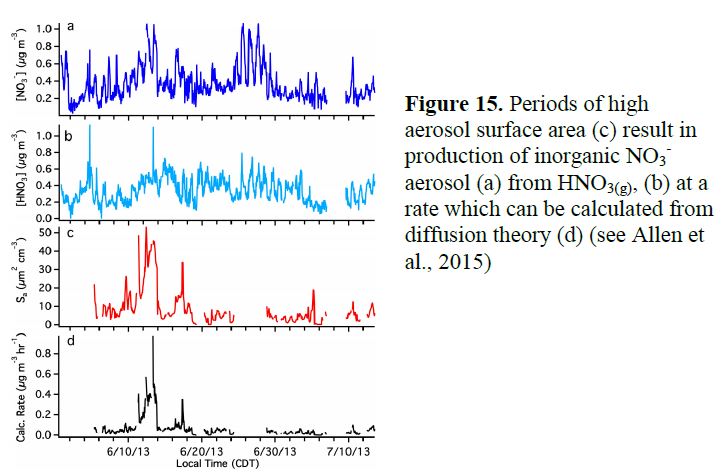
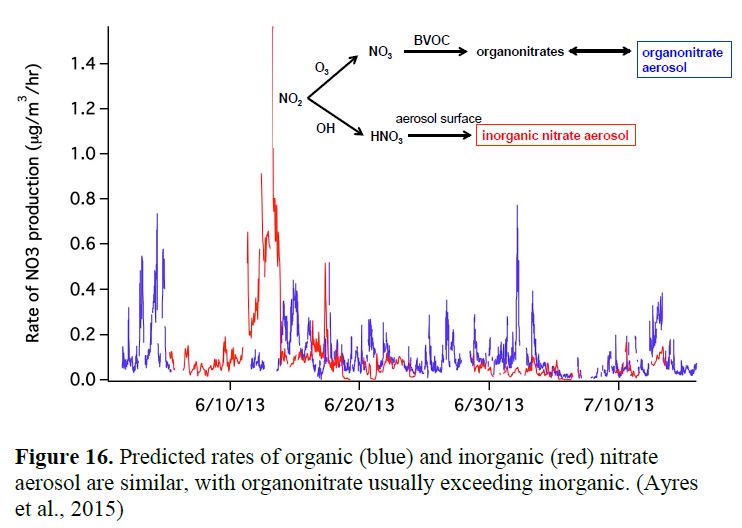
The MARGA instrument ran very well for the majority of the campaign, so we have a complete dataset on inorganic partitioning (Figure 13), from which we can determine whether aerosol is in thermodynamic equilibrium, and calculate aerosol acidity based on ion balance. We find interesting episodes of aerosol NO3- formation likely caused by dust uptake of HNO3, based on mineral cation composition (Ca2+, K+, Na+) and high aqueous aerosol loading consistent with HNO3 depletion (Figure 14). In Allen, et al. (2015), we calculate the implied rate of inorganic nitrate production via HNO3 uptake (Figure 15), and in Ayres, et al. (2015), we compare this formation rate of inorganic nitrate to organonitrate aerosol formation (addressed above in Goals 1 & 2). This comparison is shown below in Figure 16.
Conclusions:
Large, multi-investigator field studies such as SOAS require an enormous outlay of resources, but offer the enormous advantage of producing an exhaustive, comprehensive dataset that enables years of comparative analysis above and beyond answering the initial questions of the principal investigators. The four follow-on collaborations described here, involving collaborative lab experiments, analysis of aircraft data informed by our measurements at the ground, use of another group’s dataset to analyze reaction intermediates on the way to aerosol from specific oxidants in our PAM chambers, and construction of an observationally-derived overall organic aerosol budget are all examples of this, arising from this grant alone. This abundantly demonstrates the benefits of an Early Career scientist’s participation in such a large collaborative field campaign, which justifies the costs.
Our demonstration of the importance of NO3-initiated SOA formation for the nitrogen budget, and the episodic importance of inorganic nitrate aerosol formation, enable better understanding of how present-day anthropogenic NOx emissions will affect ground-level particulate matter (PM2.5) concentrations, which are detrimental to human and environmental health. With this process-level understanding of how NOx emissions are split between possible fates, we also will be better able to predict changes in surface particulate matter concentration under future changing NOx emissions.
In collaboration with other SOAS contributors (see Pye, et al.; Romer, et al.), we also have learned through combined observations and modeling in the southeastern United States that the role of nitrate chemistry cannot be fully understood by measurements of organonitrate aerosol alone. Because aerosol-phase nitrate hydrolysis can be rapid for some organonitrates, significant organic mass may be in the aerosol phase due to nitrate chemistry but no longer contain a nitrate functional group. Hence, our estimates of SOA yield from NO3 reactions may be a lower limit.
Finally, perhaps the biggest shift in thinking about atmospheric oxidation chemistry that our work at SOAS has contributed to is the recognition that NO3 oxidation of BVOC, which can have substantial health consequences because of the very high yields of aerosol formation, is not as previously thought only important during the night. Because the reactive chemistry is so rapid, in regions with fast production of NO3 radical and high BVOC, the reaction between the two can compete with photolysis losses of NO3. Thus, chemistry previously thought to be limited to the nighttime hours must be considered in predicting and understanding daytime air quality as well.
Journal Articles on this Report : 9 Displayed | Download in RIS Format
| Other project views: | All 22 publications | 11 publications in selected types | All 11 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Allen HM, Draper DC, Ayres BR, Ault A, Bondy A, Takahama S, Modini RL, Baumann K, Edgerton E, Knote C, Laskin A, Wang B, Fry JL. Influence of crustal dust and sea spray supermicron particle concentrations and acidity on inorganic NO3− aerosol during the 2013 Southern Oxidant and Aerosol Study. Atmospheric Chemistry and Physics 2015;15(18):10669-10685. |
R835399 (2014) R835399 (Final) R835409 (2014) R835409 (Final) |
Exit Exit Exit |
|
|
Ayres BR, Allen HM, Draper DC, Brown SS, Wild RJ, Jimenez JL, Day DA, Campuzano-Jost P, Hu W, de Gouw J, Koss A, Cohen RC, Duffey KC, Romer P, Baumann K, Edgerton E, Takahama S, Thornton JA, Lee BH, Lopez-Hilfiker FD, Mohr C, Wennberg PO, Nguyen TB, Teng A, Goldstein AH, Olson K, Fry JL. Organic nitrate aerosol formation via NO3 + biogenic volatile organic compounds in the southeastern United States. Atmospheric Chemistry and Physics 2015;15(23):13377-13392. |
R835399 (Final) |
Exit Exit |
|
|
Day D, Fry J, Kang H, Krechmer J, Ayres B, Keehan N, Thompson S, Hu W, Campos-Jost P, Schroder J, Stark J, DeVault M, Ziemann P, Zarzana K, Wild R, Dube W, Brown S, Jimenez J. Secondary Organic Aerosol Mass Yields from NO3 Oxidation of alpha-Pinene and Delta-Carene:Effect of RO2 Radical Fate. JOURNAL OF PHYSICAL CHEMISTRY A 2022;ePub Ahead of Print. |
R835399 (Final) |
Exit |
|
|
Draper DC, Farmer DK, Desyaterik Y, Fry JL. A qualitative comparison of secondary organic aerosol yields and composition from ozonolysis of monoterpenes at varying concentrations of NO2. Atmospheric Chemistry and Physics 2015;15(21):12267-12281. |
R835399 (Final) |
Exit Exit |
|
|
Pye HOT, Luecken DJ, Xu L, Boyd CM, Ng NL, Baker KR, Ayres BR, Bash JO, Baumann K, Carter WPL, Edgerton E, Fry JL, Hutzell WT, Schwede DB, Shepson PB. Modeling the current and future roles of particulate organic nitrates in the Southeastern United States. Environmental Science & Technology 2015;49(24):14195-14203. |
R835399 (Final) R835403 (2014) R835403 (2015) R835403 (Final) R835409 (Final) R835410 (2013) |
Exit Exit Exit |
|
|
Pye HOT, Zuend A, Fry JL, Isaacman-VanWertz G, Capps SL, Appel KW, Foroutan H, Xu L, Ng NL, Goldstein AH. Coupling of organic and inorganic aerosol systems and the effect on gas–particle partitioning in the southeastern US. Atmospheric Chemistry & Physics 2018;18(1):357-370. |
R835399 (Final) R835403 (Final) |
Exit Exit |
|
|
Romer PS, Duffey KC, Wooldridge PJ, Allen HM, Ayres BR, Brown SS, Brune WH, Crounse JD, de Gouw J, Draper DC, Feiner PA, Fry JL, Goldstein AH, Koss A, Misztal PK, Nguyen TB, Olson K, Teng AP, Wennberg PO, Wild RJ, Zhang L, Cohen RC. The lifetime of nitrogen oxides in an isoprene-dominated forest. Atmospheric Chemistry and Physics 2016;16(12):7623-7637. |
R835399 (Final) R835407 (Final) |
Exit Exit |
|
|
Washenfelder RA, Attwood AR, Brock CA, Guo H, Xu L, Weber RJ, Ng NL, Allen HM, Ayres BR, Baumann K, Cohen RC, Draper DC, Duffey KC, Edgerton E, Fry JL, Hu WW, Jimenez JL, Palm BB, Romer P, Stone EA, Wooldridge PJ, Brown SS. Biomass burning dominates brown carbon absorption in the rural southeastern United States. Geophysical Research Letters 2015;42(2):653-664. |
R835399 (2014) R835399 (Final) R835401 (2014) R835401 (Final) |
Exit Exit Exit |
|
|
Fry JL, Brown SS, Middlebrook AM, Edwards PM, Campuzano-Jost P, Day DA, Jimenez JL, Allen HM, Ryerson TB, Pollack I, Graus M, Warneke C, de Gouw JA, Brock CA, Gilman J, Lerner BM, Dubé WP, Liao J and Welti A. Secondary organic aerosol (SOA) yields from NO3 radical + isoprene based on nighttime aircraft power plant plume transects. Atmospheric Chemistry and Physics 2018; 18(16):11663-11682. |
R835399 (Final) R835877 (2018) R835877 (2019) R835877 (Final) |
Exit |
Supplemental Keywords:
ambient air, ozone, acid deposition, global climate, chemical transport, environmental chemistry, analytical, Alabama, AL, EPA Region 4;Relevant Websites:
Juliane L. Fry | Reed College ExitProgress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.

![Figure 2.Stacked cumulative BVOC loss rates sorted by oxidant: OH (green), O3(blue), NO3(red), for all BVOC (left), and monoterpenes only (right).[NO3] is based on steady-state estimate (see Ayres et al, ACP 2015); O3 concentrations from the NOAA CRDS, and OH concentrations courtesy Bill Brune.](https://cfpub.epa.gov/ncer_abstracts/images/fckimages/index.cfm?imgid=15847)

![Figure 5. Production rate of NO3 radical, based on CRDS measured NO2 and O3. This can be combined with [BVOC] to predict steady-state [NO3]ss and thus BVOC-nitrates production rates.](https://cfpub.epa.gov/ncer_abstracts/images/fckimages/index.cfm?imgid=15850)

![Figure 13. Observed aerosol fraction for each of major inorganic species (e.g. fraction of NO3= [NO3-]/([NO3-]+[HNO3]) measured by MARGA.](https://cfpub.epa.gov/ncer_abstracts/images/fckimages/index.cfm?imgid=15858)